注意:因业务调整,暂不接受个人委托测试望见谅。
FDA GUIDANCE 4
软件验证的一般原则——行业和FDA工作人员的最终指南
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】2002-01-01
- 【CCS分类】
- 【ICS分类】
FDA GUIDANCE 3
FDA审查人员和行业指南.医疗器械中所含软件上市前提交内容指南
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1998-05-28
- 【CCS分类】
- 【ICS分类】
FDA none
新鲜水果和蔬菜微生物食品安全危害最小化指南-工作草案
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1997-11-25
- 【CCS分类】
- 【ICS分类】
FDA 175.300
FDA Food Code
《食品法典》 1999年——美国公共卫生服务局食品和药物管理局的建议
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1999-01-01
- 【CCS分类】
- 【ICS分类】
FDA 97-4179
医疗器械质量体系手册:小型实体合规指南——第一版(取代《医疗器械良好制造规范手册》——FDA 97-4179)
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1996-12-01
- 【CCS分类】
- 【ICS分类】
FDA 95-4158
上市前通知510(K)医疗器械的监管要求
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1996-01-02
- 【CCS分类】
- 【ICS分类】
FDA Regulation of Medical Devices
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1995-08-01
- 【CCS分类】
- 【ICS分类】
FDA 92-4159
调查设备豁免手册
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1992-06-01
- 【CCS分类】
- 【ICS分类】
FDA Bad Bug Book
食品安全与应用营养中心-食源性致病微生物和天然毒素手册
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1992-01-01
- 【CCS分类】
- 【ICS分类】
SEPT FDA Guidance Checklist
美国食品和药物管理局(FDA)工业文件指南、美国食品和药物管理局(FDA)审查员和医疗器械中现成软件使用合规性检查表?经修订的?关于包含现成(OTS)软件的网络医疗设备的Cyersecurity的行业、FDA审查员和合规性指南
- 【发布单位或类别】 UNKNOWN其他未分类
- 【发布日期】2005-05-01
- 【CCS分类】
- 【ICS分类】
FDA CDRH-DD3
包含软件草案文件的医疗器械上市前提交内容的ODE指南
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1996-09-03
- 【CCS分类】
- 【ICS分类】
Mastering and Managing the FDA Maze
- 【发布单位或类别】 UNKNOWN其他未分类
- 【发布日期】1999-01-01
- 【CCS分类】
- 【ICS分类】
SEPT FDA 21 CFR Part 11 Checklist
FDA文件“FDA 21 CFR第11部分:电子记录;电子签名;最终规则”的证据产品清单
- 【发布单位或类别】 UNKNOWN其他未分类
- 【发布日期】2001-10-01
- 【CCS分类】
- 【ICS分类】
The FDA & Worldwide Quality System Requirements Guidebook for Medical Devices
- 【发布单位或类别】 UNKNOWN其他未分类
- 【发布日期】1996-10-01
- 【CCS分类】
- 【ICS分类】
The FDA and Worldwide Quality System Requirements Guidebook for Medical Devices;Second Edition
- 【发布单位或类别】 UNKNOWN其他未分类
- 【发布日期】2008-06-06
- 【CCS分类】
- 【ICS分类】
BS 2011-2.1FDA:1973
环境测试 测验 检测Fda 随机振动-宽带再现性高
- 【发布单位或类别】 GB-BSI英国标准学会
- 【发布日期】1973-11-15
- 【CCS分类】
- 【ICS分类】
FDA Guidance for the Content of Pre-market Submissions for Software Contained in Medical Devices-Evidence Product Checklist
- 【发布单位或类别】 US-FDA美国食品药物管理局
- 【发布日期】1998-05-01
- 【CCS分类】
- 【ICS分类】
General Principles of Software Validation-Final Guidance for Industry and FDA staff-Evidence Product Checklist
- 【发布单位或类别】 UNKNOWN其他未分类
- 【发布日期】2002-01-11
- 【CCS分类】
- 【ICS分类】
Evidence Product Checklist for FDA Guidance for the Content of Pre-market Submissions for Software Contained in Medical Devices;May 11;2005
- 【发布单位或类别】 UNKNOWN其他未分类
- 【发布日期】2005-05-11
- 【CCS分类】
- 【ICS分类】
实验仪器




测试流程
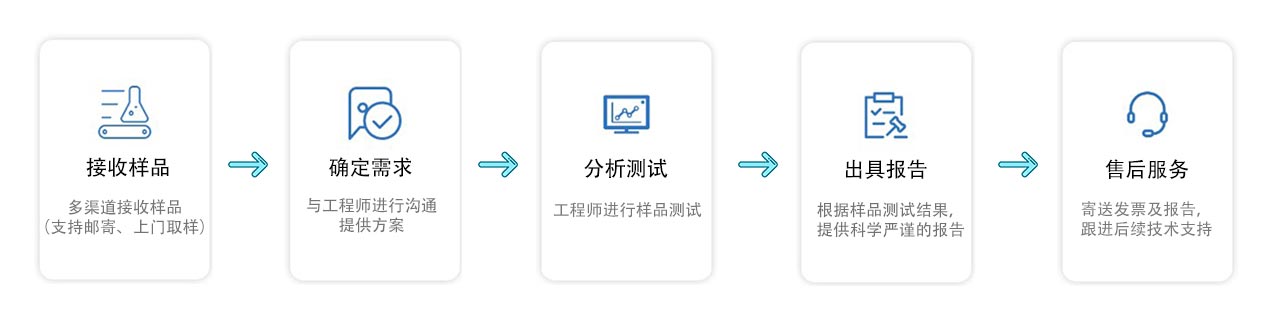
注意事项
1.具体的试验周期以工程师告知的为准。
2.文章中的图片或者标准以及具体的试验方案仅供参考,因为每个样品和项目都有所不同,所以最终以工程师告知的为准。
3.关于(样品量)的需求,最好是先咨询我们的工程师确定,避免不必要的样品损失。
4.加急试验周期一般是五个工作日左右,部分样品有所差异
5.如果对于(美国fda检测检测标准)还有什么疑问,可以咨询我们的工程师为您一一解答。
上一篇: esd检测参考检测标准
下一篇: 病毒检测地方检测标准
- 新冠检测调低检测标准阅读:16
- 噪声检测行业检测标准阅读:11
- 房屋节能检测的检测标准阅读:12
- 面料国标检测PH检测标准阅读:9
- 渗碳深度检测方法检测标准阅读:8
- 废水cod检测方法检测标准阅读:10
- 管道缺陷检测的检测标准阅读:12
- 机油检测测评检测标准阅读:17
- 车灯检测ece检测标准阅读:18
- 检测服务流程视频检测标准阅读:13
-
服务保障 一对一品质服务
-
定制方案 提供非标定制试验方案
-
保密协议 签订保密协议,严格保护客户隐私
-
全国取样/寄样 全国上门取样/寄样/现场试验





